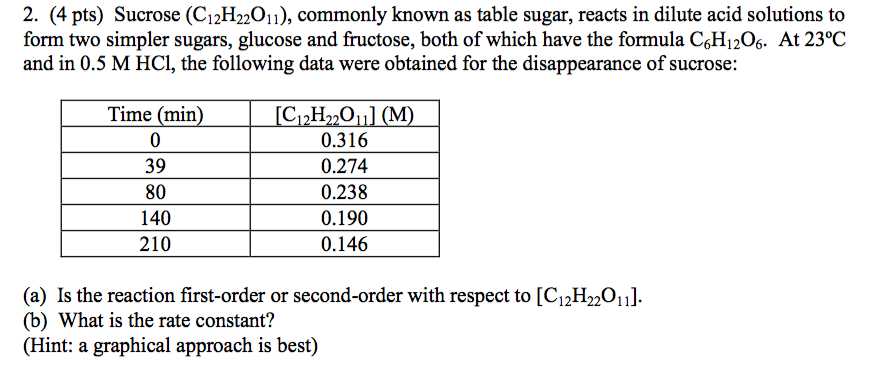
Show transcribed image text 2. (4 pts) Sucrose (C12H22011), commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula CsH1206. At 23°C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) 30 80 140 210 0.316 0.274 0.238 0.190 0.146 (a) Is the reaction first-order or second-order with respect to [C12H20ul (b) What is the rate constant? Hint: a graphical approach is best)
2. (4 pts) Sucrose (C12H22011), commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula CsH1206. At 23°C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) 30 80 140 210 0.316 0.274 0.238 0.190 0.146 (a) Is the reaction first-order or second-order with respect to [C12H20ul (b) What is the rate constant? Hint: a graphical approach is best)






